 |
|
Neurobiology of olfactory learning and individual recognition
Hideto Kaba
Professor, Department of Physiology, Medical School
Outline of Research
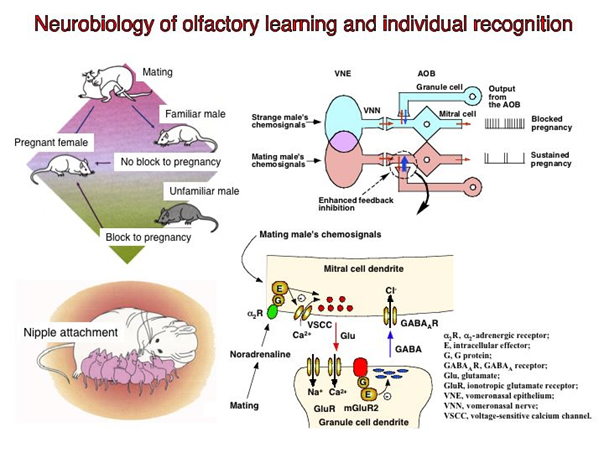
Olfaction is the dominant sensory modality for most animals and olfactory communication plays a pivotal role in conveying information about individuality. Chemical signals that convey information between members of the same species are commonly termed pheromones. Most mammalian species possesses two major olfactory systems, the main olfactory system and the vomeronasal system that should be viewed as complementary rather separate pathways for olfactory communication. Our understanding of this form of communication has grown rapidly over the last fifteen years since the identification of the first olfactory receptor genes. The subsequent cloning of genes for rodent vomeronasal receptors, which are important in pheromone detection, has revealed an unexpected diversity of around 250 receptors belonging to two structurally different classes, V1Rs and V2Rs.
A characteristic feature of all animals is the ability to learn and store information as a result of experience. Many different forms of learning and memory are found, both between different species and within the nervous system of an individual. We have been studying the synaptic and molecular mechanisms of olfactory learning in three specialized contexts that occur during sensitive periods of enhanced neural plasticity. It is because such learning is often very robust, with large neural changes that are easy to measure. These include (1) individual recognition memory of male pheromonal signals by female mice, (2) olfactory learning in rats, and (3) maternal memory in rats. All three forms of olfactory learning are associated with neural changes in the olfactory bulb (OB) at the first stage of sensory processing. These changes require the association of the olfactory and somatosensory signals in the OB. We are currently focusing on the role of membrane receptors and their down-stream cascades in the three forms of olfactory learning.
Prof. Hideto Kaba
Education
Veterinary Medical Science, Faculty of Agriculture, Kagoshima University -------- DVM--- 1974
Nutritional Chemistry, Graduate School of Nutrition, University of Tokushima ----- PhD--- 1982
Positions
Instructor, Department of Physiology, Kochi Medical School --------------------------1980-1988
Associate Professor, Department of Physiology, Kochi Medical School---------------- 1988-1995
Professor, Veterinary Physiology, Veterinary Medical Science,
Faculty of Agriculture, Kagoshima University -------------------------------------------1995-1997
Professor, Department of Physiology, Kochi Medical School ---------------------------1997-Present
Executive Editor, Chemical Senses -----------------------------------------------------2003-2008
Adjunct Professor, Division of Adaptation Development, Department of
Developmental Physiology, National Institute for Physiological Sciences---------------2003-2009
President, The Japanese Association for the Study of Taste and Smell ----------------2009-Present
Representative papers
(1) Olfactory recognition: a simple memory system.
Brennan P, Kaba H, Keverne EB.
Science. 1990 Nov 30;250(4985):1223-6.
(2) Induction of an olfactory memory by the activation of a metabotropic glutamate receptor.
Kaba H, Hayashi Y, Higuchi T, Nakanishi S.
Science. 1994 Jul 8;265(5169):262-4.
(3) Olfactory imprinting in mammals.
Kaba H
In: Comparative Social Cognition, Watanabe S, Tsujii T, Keenan JP (eds), Keio University Press, 2007, pp 79-101.
|
|
|
Identification of phage receptors and elucidation of their functions
Shigenobu Matsuzaki
Associate Professor, Department of Microbiology and Infection, Medical School
Outline of Research
To initiate successful infection, tailed bacteriophage (phage) adsorb to the host bacteria through a specific and strong interaction between the ligand at the tip of the phage tail and a receptor molecule on the bacterial cell surface. In this study, we will try to develop a novel method for bacterial detection using the specific interaction between the phage ligand and the bacterial receptor. We will also try to clarify the role of the bacterial receptor molecule (one bacterial surface molecule) in bacterial infection in an animal model.
(1) Identification and purification of the phage ligand molecule. The phage ligand gene will be detected by the analysis of the nucleotide sequence of the phage genome. The gene will be cloned into a plasmid vector, and the gene product expressed in Escherichia coli, after which it will be purified using tools such as a His-tag column. The host bacterium will be treated with the purified ligand, and the binding of the ligand to the bacterium will be detected using a chemiluminescent reaction, agglutinative reaction, etc.
(2) Identification and purification of the receptor molecule. A receptor-defective mutant bacterium will be sought among phage-resistant mutants. The receptor molecule can then be detected in a comparison of the cell-surface molecules of the mutant with those of the parental strain. The putative molecule will be confirmed to be the receptor in a competition experiment for phage adsorption.
(3) Examination of the role of the bacterial surface molecule (the phage receptor molecule) in bacterial infection in an animal model. The role of the receptor molecule in the bacterial infection of mammal cells will be examined by comparing the infectivity of the wild-type bacterium with that of the receptor-defective bacterium. The effects of the receptor molecule itself on mammalian cells will also be examined.
Dr. Shigenobu Matsuzaki
Graduated from Department of Literature and Science, Yamaguchi University, Japan, in1977.
Hiroshima University Postgraduate School from 1978 to 1985.
Research associate at Kochi Medical School from 1986 to 2003
International researcher of the Ministry of Education at Harvard Medical School, USA, from 1996 to 1997.
Associated Professor at Kochi University Medical School from 2003 to 2010.
Received Ph.Ds (1986 from Hiroshima University; 2003 from Kochi Medical School).
Selected Papers
(1) In silico and in vivo evaluation of bacteriophage φEF24C, a candidate for treatment of Enterococcus faecalis infections. Uchiyama J, Rashel M, Takemura I, Wakiguchi H, Matsuzaki S. Appl Environ Microbiol. 2008 Jul;74(13):4149-63. Epub 2008 May 2.
(2) Efficient elimination of multidrug-resistant Staphylococcus aureus by cloned lysin derived from bacteriophage φMR11. Rashel M, Uchiyama J, Ujihara T, Uehara Y, Kuramoto S, Sugihara S, Yagyu K, Muraoka A, Sugai M, Hiramatsu K, Honke K, Matsuzaki S. J Infect Dis. 2007 Oct 15;196(8):1237-47. Epub 2007 Sep 11.
(3) Experimental protection of mice against lethal Staphylococcus aureus infection by novel bacteriophage φMR11. Matsuzaki S, Yasuda M, Nishikawa H, Kuroda M, Ujihara T, Shuin T, Shen Y, Jin Z, Fujimoto S, Nasimuzzaman MD, Wakiguchi H, Sugihara S, Sugiura T, Koda S, Muraoka A, Imai S. J Infect Dis. 2003 Feb 15;187(4):613-24. Epub 2003 Feb 7. |
|
|
A study on axon guidance molecules in the ventral telencephalon
Shinji Hirano
Associate Professor, Department of Neurobiology & Anatomy, Medical School
In the ventral telencephalon, there are several major neural pathways, such as corticothalamic pathway, corticospinal pathway, thalamocortical pathway, and strionigral pathways, that are essential in neural activity of the cerebral cortex. Previous studies suggested possible mechanisms in formation of these neural circuits: they include hypothetical guide-post cells and morphoregulatory molecules, such as Pax6 and slit-1. By now, I have discovered OL-protocadherin, a cell adhesion molecule, in the mouse brain and have shown that this molecule is involved in striatal axon growth, that process in turn plays important roles in the formation of corticospinal and thalamocortical pathways. Although many studies on neural circuit formation in the ventral telencelphalon has been done, mechanism are not fully understood. For example, it remains unknown how striatal axons form a thick axon bundle when they progress in the globus pallidus and how thalamocortical axons make turn at the boundary region and progress toward the cortex .
To reveal molecular mechanisms of neural circuit formation in this region, I will attempt to identify axon guidance molecules and analyze them in the present study. First, I will find putative guidance molecules that are expressed in this region, and then analyze their functions in culture systems and in the mutant mice brain. Here, I will focus on certain molecules such as semapholines/plexins repellent molecules and protocadherin cell adhesion molecules. Through analyses of these molecules, I will elucidate novel mechanisms of axon guidance in this region.
Dr. Shinji Hirano
1987 Graduation from Kyoto Univ. Fac. Science; 1992 Kyoto Univ. PhD.; National Institute for Basic Biology, Research Assistant; University of Southern California Doheny Eye Institute, postodoctral fellow; Human Frontier Science Program, long-term fellow; Aichi Human Service Center Instituite for Developmetal Research, Researcher; JST PRESTO, researcher; RIKEN Center for Developmental Biology Researcher; 2008 till present Kochi Univ.
Selected papers
(1) Pioneers in the ventral telencephalon: The role of OL-protocadherin-dependent striatal axon growth in neural circuit formation.
Hirano S.
Cell Adh Migr. 2007 Oct;1(4):176-8.
(2) OL-Protocadherin is essential for growth of striatal axons and thalamocortical projections.
Uemura M, Nakao S, Suzuki ST, Takeichi M, Hirano S.
Nat Neurosci. 2007 Sep;10(9):1151-9.
(3) Expression of a novel protocadherin, OL-protocadherin, in a subset of functional systems of the developing mouse brain.
Hirano S, Yan Q, Suzuki ST.
J Neurosci. 1999 Feb 1;19(3):995-1005.
|
|
|
Mechanisms for expression of selectin ligands on KL-6/MUC1
Akihito Yokoyama
Professor, Department of Hematology and Respiratory Medicine, Medical School
Overview of our research
Previously, we reported KL-6, a sialylated sugar chain exclusively expressed on MUC1 mucin as a diagnostic and prognostic circulating marker for interstitial pneumonia, ALI/ARDS, and lung cancer. Serum KL-6 level has been approved by Japan’s Health Insurance Program as a diagnostic marker for interstitial lung diseases, and more than million samples are now examined per year. More recently, we demonstrated that KL-6/MUC1 was an independent predictor for DIC in patients with ARDS. Increases in circulating levels of KL-6/MUC1 preceded DIC development, suggesting that KL-6/MUC1 may be involved in the pathophysiology of the coagulation abnormality in ARDS patients.
The selectins, a family of adhesion molecules expressed on various cell surfaces, mediate immunological and inflammatory cell-cell reactions through selectin ligands. Anti-selectin treatment has shown favorable outcomes in several experimental ALI/ARDS models. We reported a submolecule of KL-6/MUC1 on which selectin ligands are expressed. We constructed an ELISA system to measure KL-6/MUC1 carrying selectin ligand (sialyl Lewisª) in sera and designated the molecule as “SLAK”. The circulating level of SLAK was an independent prognostic factor in patients with lung adenocarcinoma, and higher levels of serum SLAK were observed in lung adenocarcinoma patients with distant metastasis.
According to a recent review, carcinoma mucin-selectin interaction induces platelet aggregation. This interaction is involved in the mechanism of Trousseau’s syndrome, which is a carcinoma-induced coagulopathy. Many sugar chains, including selectin ligands, are conjugated to mucins, which can activate immune responses, cell-cell responses, and cell-cell adhesions via the sugar chains. Heavily glycosylated carcinoma mucins injected into mice can act as selectin ligands and this interaction results in the formation of disseminated platelet-rich microthrombi. Moreover, the mucin-selectin mediated intravascular coagulation does not depend on the existence of tissue factor or endotoxin.
In the present study, we hypothesized that circulating KL-6/MUC1 with selectin ligands may be associated with coagulation abnormality in a non-malignant disease, ALI/ARDS. To clarify this, serum levels of KL-6/MUC1 with selectin ligands will be measured and compared with the level of P-selectin glycoprotein ligand-1 (PSGL-1), the mucin-like glycoprotein identified as a circulating functional ligand for P-selectin. If increases in circulating KL-6/MUC1 carrying selectin ligands are more likely to be causally related to intravascular coagulation when compared with the entire amount of KL-6/MUC1, we would like to disclose the mechanisms for synthesis of KL-6/MUC1 carrying selectin ligands.
Prof. Akihito Yokoyama
Professional Training and Employment
1986-1988 Research Fellow, Division of Immunology, Department of Pathology, University of Chicago, Chicago, U.S.A.
1988-1990 Instructor, Department of Internal Medicine, Toyama Medical
Pharmaceutical University, Toyama, Japan
1991-1999 Instructor, Department of Internal Medicine II, Ehime University
School of Medicine, Ehime, Japan
2000-2002 Lecturer, Department of Internal Medicine II, Ehime University
School of Medicine, Ehime, Japan
2003-2004 Lecturer, Department of Molecular and Internal Medicine,
Graduate School of Biomedical Sciences, Hiroshima University
Hiroshima, Japan
2005-2006 Associate Professor, Department of Molecular and Internal
Medicine, Graduate School of Biomedical Sciences,
Hiroshima University, Hiroshima, Japan
2007-present Professor and Chairman, Department of Hematology and
Respiratory Medicine, Kochi University, Kochi, Japan
Selected Papers
1.Circulating KL-6/MUC1 as an independent predictor for disseminated intravascular coagulation in acute respiratory distress syndrome. Nakashima T, Yokoyama A, Ohnishi1 H, Hamada H, Ishikawa N, Haruta Y, Hattori N, Tanigawa K, Kohno N.
J Intern Med, 2008;263:432-9
2. Suppressor of cytokine signaling1 inhibits pulmonary inflammation and fibrosis. Nakashima T, Yokoyama A, Ohnari Y, Syoda H, Haruta Y, Hattori N, Naka T, Kohno N.
J Allergy Clin Immunol, 2008;121:1269-76.
3.Airflow Limitation in Smokers is Associated with Subclinical Atherosclerosis. Iwamoto H, Yokoyama A, Kitahara Y, Ishikawa N, Haruta Y, Yamane K, Hattori N, Hara H, Kohno N.
Am J Respir Crit Care Med. 2009;179:35-40 |
|
|
Roles of brain phospholipids turnover in the central regulation of sympatho-adrenomedullary outflow
Takahiro Shimizu
Associate Professor, Department of Pharmacology, Medical School
Outline of Research
In plasma membrane, there are many kinds of proteins [receptors, GTP-binding proteins, phospholipase C (PLC), etc], which change extracellular stimuli to second messengers in cells. Besides, the membrane also functions as sources of transmitters, therefore, the plasma membrane plays important roles in biological activities.
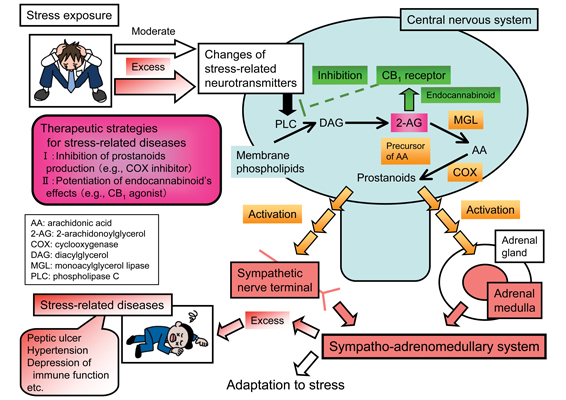
Focusing on prostanoids, transmitters produced from the plasma membrane phospholipids, we have been examining the mechanisms of physiological and pathophysiological responses to stressors in relationship to the sympatho-adrenomedullary (SA) system, which plays an important role in the responses. To date, we have reported the involvement of brain prostanoids in the activation of SA outflow (Shimizu et al., Eur J Pharmacol 2006;541:152-7; Lu et al., Eur J Pharmacol 2008;590:177-84; Sasaki et al., Eur J Pharmacol 2008;592:81-6; Shimizu and Yokotani, Eur J Pharmacol 2009;606:77-83; Eur J Pharmacol 2009;611:30-4).
Prostanoids are active metabolites of arachidonic acid, therefore, we examined the mechanisms of brain arachidonic acid/prostanoids production involving in the activation of SA outflow. Arachidonic acid is widely known to be released from phospholipids mainly by phospholipase A2 (PLA2), however, we reported the involvement of brain PLC rather than PLA2 in the activation of SA outflow. Namely, (1)brain phospholipids are metabolized by PLC and diacylglycerol lipase to 2-arachidonoylglycerol (2-AG), which involving in the activation as a source of brain arachidonic acid/prostanoids (Shimizu et al., Eur J Pharmacol 2004;499:99-105; Eur J Pharmacol 2005;514:151-8; Eur J Pharmacol 2007;571:138-44; Eur J Pharmacol 2010;641:54-60; Eur J Pharmacol 2011;658:123-31; Eur J Pharmacol 2012;691:93-102; Shimizu and Yokotani, Eur J Pharmacol 2008;582:62-9). In addition, we reported that (2) brain 2-AG also plays an inhibitory role in the activation of SA outflow as an endocannabinoid (eCB), an endogenous ligand for cannabinoid CB1 receptors (Shimizu and Yokotani, Eur J Pharmacol 2008;582:62-9; Shimizu et al., Eur J Pharmacol 2010;641:54-60; Eur J Pharmacol 2011;658:123-31). These reports indicate bidirectional roles of brain 2-AG, (1) a stimulatory role as a source of arachidonic acid/prostanoids and (2) an inhibitory role as a brain eCB, in the activation of SA outflow (Shimizu et al., J Pharmacol Sci 2013;121:157-71).
The purpose of this research is clarifying the central regulation mechanisms of SA outflow in relationship to transmitters produced from the brain plasma membrane phospholipids, especially prostanoids and eCB, and receptors for the transmitters. The SA system plays an important role in physiological and pathophysiological responses to stressors and also modulates cardiovascular and immune functions, therefore, excess activation of the system may contribute to the development of “stress-related diseases” including hypertension. In order to establish complete therapy for the diseases, it is necessary to create therapeutic strategies targeted to the central nervous system, regulating the SA system. The outcomes of this research may contribute to clarifying the mechanisms for adaptation to stressors and creating complete therapy for the stress-related diseases.
Dr. Takahiro Shimizu
Education2001 Bachelor of Pharmacy, Hokkaido University, Japan
2003 Master of Pharmacy, Hokkaido University, Japan
2008 Ph.D. of Medicine, Kochi University, Japan
Career
2003 Assistant Professor, Department of Pharmacology, Kochi Medical School, Japan
2003-2012 Assistant Professor, Department of Pharmacology, Kochi University School of Medicine, Japan
2012-present Associate Professor, Department of Pharmacology, Kochi University School of Medicine, Japan
Award
2012 Annual Meeting Outstanding Presentation Award
(The 85th Annual Meeting of The Japanese Pharmacological Society)
Selected Papers
1) Tanaka K, Shimizu T, Yanagita T, Nemoto T, Nakamura K, Taniuchi K, Dimitriadis F, Yokotani K, Saito M. Brain RVD-haemopressin, a haemoglobin-derived peptide, inhibits bombesin-induced central activation of adrenomedullary outflow in the rat. Br J Pharmacol, 171, 202-213, 2014
2) Shimizu T, Tanaka K, Yokotani K. Stimulatory and inhibitory roles of brain 2-arachidonoylglycerol in bombesin-induced central activation of adrenomedullary outflow in rats. J Pharmacol Sci, 121, 157-171, 2013
3) Shimizu T, Tanaka K, Nakamura K, Taniuchi K, Yokotani K. Brain phospholipase C, diacylglycerol lipase and monoacylglycerol lipase are involved in (±)-epibatidine-induced activation of central adrenomedullary outflow in rats. Eur J Pharmacol, 691, 93-102, 2012
4) Shimizu T, Yokotani K. Bidirectional roles of the brain 2-arachidonoyl-sn-glycerol in the centrally administered vasopressin-induced adrenomedullary outflow in rats. Eur J Pharmacol, 582, 62-69, 2008
5) Shimizu T, Uehara T, Nomura Y. Possible involvement of pyruvate kinase in acquisition of tolerance to hypoxic stress in glial cells. J Neurochem, 91, 167-175, 2004 |
|
|
T cell recognition taking place on lipid rafts
Keiko UDAKA
Professor, Department of Immunology, Medical School
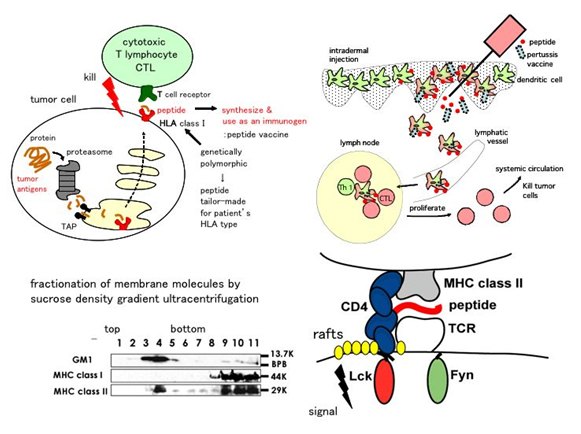
T cells recognize not only the antigenic MHC (Major Histocompatibility Complex)-peptide complexes (MHC-pep) but also those loaded with self peptides. When recognizing antigenic MHC-pep T cells are activated and execute the functions to eliminate pathogens. In contrast, when recognizing self MHC-pep T cells are silenced and act to maintain self-tolerance. These two contrasting responses are initiated by a subtle difference in the affinity, i.e. less than 10-fold on a single molecular basis, between T cell receptor (TCR) and MHC-pep. TCR and a number of membrane molecules involved in signal reception are thought to gather on the plasma membrane and signal differentially according to the affinity. Physical understanding of this signal reception is yet to be investigated. Our main interest resides in finding a physical basis of self-nonself differentiation.
Since antigen recognition by TCR takes place in lipid rafts on the plasma membrane we are investigating how a change of physical properties such as glycosylation and fluidity of lipid affect antigen recognition. Using gene KO mouse models we hope to find a way to restrain T cell responses against particular antigens but not to pathogens.
We are also interested in the mechanism how recognition of senescent tissues takes place in lipid rafts. Finally, we are developing a peptide-based immunotherapy against tumors and virus infected cells by taking advantage of the raft associated recognition of antigens by T cells.
Prof. Keiko Udaka
Educational history
1982 Bachelor of Medicine, School of Medicine, Ehime University, M.D.
1986 M.D. PhD, Graduate school of Medicine, Ehime University
Occupational history
1985-88 Postdoctoral fellow, Department of Microbiology, University of Pennsylvania
1988-91 Postdoctoral fellow,
Center for Cancer Research, Massachusetts Institute of Technology
1991-93 Research fellow, Humboldt Foundation
Department of Immunogenetics, Max-Planck Institute of Biology
1994-94 Assistant professor with no stipend, Department of Immunology, Juntendo University
1994-99 Assistant professor, Department of Biophysics, Kyoto University
1999-02 Associate professor, Department of Biophysics, Kyoto University
2003-03 Professor, Department of Immunology, Kochi Medical School
2003- Professor, Department of Immunology, School of Medicine, Kochi University
Selected papers
(1) A naturally occurring peptide recognized by alloreactive CD8+ cytotoxic T lymphocytes in association with a class I MHC protein.
Keiko Udaka, Theodore J. Tsomides, Herman N. Eisen.
Cell. 69, 989-998, 1992.
(2) Empirical evaluation of a dynamic experiment design method for prediction of MHC class I-binding peptides.
Keiko Udaka, Hiroshi Mamitsuka, Yukinobu Nakaseko and Naoki Abe.
J Immunol, 169, 5744-5753, 2002
(3) Lipid-mediated presentation of MHC class II molecules guides thymocytes to the CD4 lineage.
Satoshi Komaniwa, Hiroshi Hayashi, Hiroshi Kawamoto, Satoshi B. Sato, Tomokatsu Ikawa, Yoshimoto Katsura, and Keiko Udaka
Eur J Immunol, 39, 1-7, 2009 |
|
|
Intermolecular network in membrane microdomains
Koichi Honke
Professor, Department of Biochemistry, Medical School
Outline of Research
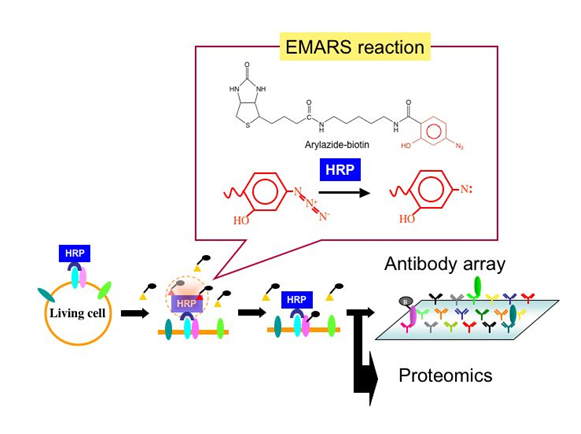
Important biological events associated with plasma membranes, such as signal transduction, cell adhesion, and protein trafficking, are mediated through the membrane microdomains. However, it is difficult to assess the issue of how they assemble under physiological conditions. We developed a new approach to identify partners of a given molecule on the cell surface in living cells. The important feature of this system, termed as Enzyme-Mediated Activation of Radical Source (EMARS), is that activation of crosslinking reagent arylazide-biotin tag can be accomplished by horseradish peroxidase (HRP). By using this method, we found that many kinds of receptor tyrosine kinases are associated with b1 integrin whereas a few receptor tyrosine kinases are associated with ganglioside GM1 in HeLa S3 cells. This system is a comprehensive approach to identify biochemically interactions between cell surface molecules under living conditions.
We aim at elucidation of the intermolecular network in the plasma membranes from a variety of patological cells and development of innovative therapy targeting molecular interactions by means of the EMARS method and the monoclonal antibody production for membrane microdomains. We already found novel molecular interactions in the plasma membranes that had been unable to find by the conventional methods. By clarifying physiological roles of those interactions, unexpected biological control systems may be revealed.
Prof. Koichi Honke
Education
1977-1983 Hokkaido University Medical School, Sapporo, Japan
1983-1986 Department of Biochemistry, Cancer Institute, Hokkaido University Medical School, Japan
Career
1987-1995 Research Associate to Lecturer at the Deapartment of Biochemistry, Cancer Institute, Hokkaido University Medical School, Japan,
1995-1999 Chief Researcher at the Osaka Medical Center for Maternal and Child Health, Japan
1999-2003 Associate Professor at the Department of Biochemistry, Osaka University Medical School, Japan
2003-present Professor at the Department of Biochemistry, Kochi University Medical School, Japan
2008-present Director at the Kochi System Glycobiology Center, Kochi University Medical School, Japan
Award
1997 JB Award (Japanese Biochemical Society)
Selected papers
1) Biochemical visualization of cell surface molecular clustering in living cells.
Kotani N, Gu J, Isaji T, Udaka K, Taniguchi N, Honke K.
Proc Natl Acad Sci U S A. 2008 May 27;105(21):7405-9.
2) Dysregulation of TGF-beta1 receptor activation leads to abnormal lung development and emphysema-like phenotype in core fucose-deficient mice.
Wang X, Inoue S, Gu J, Miyoshi E, Noda K, Li W, Mizuno-Horikawa Y, Nakano M, Asahi M, Takahashi M, Uozumi N, Ihara S, Lee SH, Ikeda Y, Yamaguchi Y, Aze Y, Tomiyama Y, Fujii J, Suzuki K, Kondo A, Shapiro SD, Lopez-Otin C, Kuwaki T, Okabe M, Honke K, Taniguchi N.
Proc Natl Acad Sci U S A. 2005 Nov 1;102(44):15791-6
3) Paranodal junction formation and spermatogenesis require sulfoglycolipids.
Honke K, Hirahara Y, Dupree J, Suzuki K, Popko B, Fukushima K, Fukushima J, Nagasawa T, Yoshida N, Wada Y, Taniguchi N.
Proc Natl Acad Sci U S A. 2002 Apr 2;99(7):4227-32. |
|
|
New therapeutic approach to kidney diseases by regenerative medicine
Yoshio Terada
Professor, Department of Endocrinology, Metabolism and Nephrology, Medical School
Outline of the projects
Background:
The molecular basis of the events leading to tubular regeneration after AKI (acute kidney injury) is only partially established. The mechanisms that lead to renal cell proliferation and regeneration must be better understood before novel therapeutic strategies for the treatment of ischemic AKI can be explored. Evidence has suggested that regeneration processes may recapitulate developmental processes in order to restore organ or tissue function. Regeneration processes may be similar to developmental processes. Embryonic genes such as Wnt-4, Ets-1, and Delta-Notch pathway are markedly induced in the mature kidney after ischemic renal injury and apparently play crucial roles in the regeneration and repair of the organ.
1) Dedifferentiation of renal tubular cell
We have reported that embryonic genes such as Wnt-4, Ets-1, and Delta-Notch pathway are markedly induced in the mature kidney after ischemic renal injury. We and other researchers have called this phenomena as“dedifferentiation”of renal tubular cells. We proposed that the dedifferentiation of renal tubular cells after AKI plays a key role in regeneration of renal tubular cells.
2) Regenerative medicine of renal disease using growth factors or genes
We also examine the possible approach of regeneration using growth factors or gene transfer. Some growth factors such as hepatocyte growth factor or erythropoietin cause proliferation of renal tubular cells and prevent apoptpsis. These growth factors may be potency for regeneration of renal tubular cells in vivo. We are planning to examine the effects these factors in AKI.
3) Regenerative medicine of renal disease using iPS cells and ES cells
We already reported that ES cells which were stably transfected with Wnt-4 gene can differentiate renal tubular cells in presence of Activin A and HGF (hapatocyte growth factor). It may be possible to differentiate iPS cells to renal tubular cells using same methodology. We will examine these approachs for regenerative medicine of renal disease
Prof. Yoshio Terada
Graduate of Tokyo Medical &Dental University (1984.3)
Visiting fellow, Laboratory of Kidney Electrolyte, and Metabolism,
National Institutes of Health (1988.1-1991.3)
Assistant Professor of Homeostasis medicine &Nephrology,
Tokyo Medical &Dental University (1994.11-2002.3)
Associate Professor of Homeostasis medicine &Nephrology,
Tokyo Medical &Dental University (2002.4-2008.3)
Professor and Chairman, Department of Endocrinology,
Metabolism and Nephrology, Kochi Medical School, Kochi University (2008.4-)
Selected Papers
- Terada Y, Tomita K, Nonoguchi H, Yang T, Marumo F: Different localization and r egulation of two types of vasopressin receptor messenger RNA in microdissected rat nephron segments using reverse transcription polymerase chain reaction. J Clin Invest 92: 2339-2345, 1993
- Terada Y, Tomita K, Homma MK, Nonoguchi H, Yang T, Yamada T, Yuasa Y, Krebs EG, Marumo F: Sequential activation of raf-1 kinase, MAP kinase kinase, MAP kinase, and S6 kinase by hyperosmolality in renal cell. J Biol Chem 269: 31296-31301, 1994
- Taniguchi K, Kohsaka H, Inoue N, Terada Y, Ito H, Hirokawa K, Miyasaka N: Introduction of p16INK4a sequence gene as a novel therapeutic strategy for the treatment of rheumatoid arthritis. Nature Med 5: 760-767, 1999
|
|
|
Crosstalk between epidermis and immunocytes to develop dermatitis after disruption of permeability barrier.
Shigetoshi Sano
Professor, Department of Dermatology, Medical School
Outline of Research
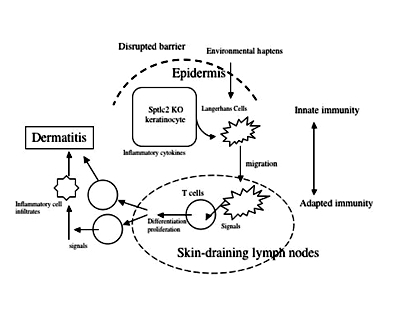
The permeability barrier mainly resides in the cornified layer of epidermis, representing a skin protecting mechanism from protrusion of environmental chemicals including antigenic haptens. Skin barrier disruption occurs following mechanical or physical perturbation, such as scratching or detergent insults. It is well recognized that barrier disruption is associated with inflammatory skin diseases, including atopic dermatitis (AD) and psoriasis. The cornified layer of AD patients demonstrated a decrease in ceramides, which is the major component of intercellular lipids required for barrier function. More recent discovery regarding mutations in the filaggrin genes from patients with AD and bronchial athma underlined the importance of barrier function to protect allergic risk, since disrupted barrier permits hapten allergens to be immunologically sensitized. However, it is still unknown whether the barrier disruption affects skin immunity directly. To address this, we established a conditional knockout mouse whose epidermis was devoid of the gene encoding the rate-limiting enzyme for de novo synthesis of ceramides (sptlc2 cKO). This mutant mouse showed a delayed recovery from barrier disruption and then developed dermatitis. Furthermore, they showed immunological alterations, including activation of Langerhans cells and propagation of Th17 cells in the skin-draining lymph nodes, suggesting that barrier disruption directly affect skin immunity with a skewed Th17 response.
Prof. Shigetoshi Sano
Education
M.D. in Ehime University Medical School (Medicine) 1983
Ph.D. in Osaka University Graduate School of Medicine (Immunology) 1988
Postdoc in Albert Einstein Medical College (Microbiology and immunology) 1988-1992
Positions
Since 2007 Professor of Dermatology, Kochi Medical School, Kochi University
2006-2007 Associate Professor, Department of Dermatology, Osaka University Medical School.
2005-2006 Assisstant Professor, Department of Dermatology, Osaka University Medical School.
2004-2005 Director of Dermatology, Sumitomo Hospital, Osaka
2003-2004 Visiting Assisstant Professor, Department of Carcinogenesis, MD Anderson Cancer Center
1994-2003 Assisstant Professor, Department of Dermatology, Osaka University Medical School.
1992-1994 Clinical Director of Dermatology, Sakai Municipal Hospital, Osaka.
1983-1984 Resident in Osaka University Hospital (Dermatology).
Selected Papers
1) Impact of Stat3 activation upon skin biology: a dichotomy of its role between homeostasis and diseases. Sano S, Chan KS, DiGiovanni J.
J Dermatol Sci. 2008 Apr;50(1):1-14.
2) Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, Itami S, Nickoloff BJ, DiGiovanni J.
Nat Med. 2005 Jan;11(1):43-9.
3) Keratinocyte-specific ablation of Stat3 exhibits impaired skin remodeling, but does not affect skin morphogenesis. Sano S, Itami S, Takeda K, Tarutani M, Yamaguchi Y, Miura H, Yoshikawa K, Akira S, Takeda J. EMBO J. 1999 Sep 1;18(17):4657-68.
|
|
|
Non-coding RNA mediated regulation of cellular signaling
Shuji Sakamoto
Assistant Professor, Laboratory of Molecular Biology, Science Research Center
Outline of Research
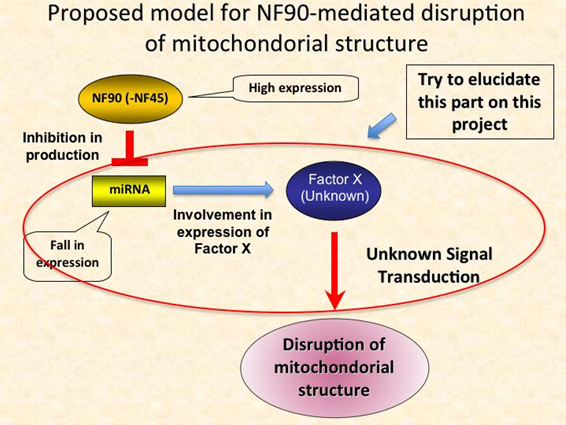
MicorRNAs (miRNAs) are a class of non-coding small RNA that function as regulators for eukaryotic gene expression. Their functional significance has been established in various cellular events like proliferation, apoptosis, and development. Recently, we have found that Nuclear Factor 90 (NF90) and it’s binding partner, NF45 complex (NF90-NF45), which is a nuclear double strand RNA binding protein, participates in negative regulation of miRNA biogenesis (MCB 2009 29 (13) 3754-69). This finding raises the possibility that NF90(-NF45) involves in various cellular process via their function as a regulator in miRNA biogenesis. To examine this feasibility, we have investigated phenotype of NF90 transgenic (Tg) mice. From this analysis, it has been clear that overexpressed NF90 impairs mitochondorial structure in muscles, resulting in losses of heart function and skeletal muscle formation, which would lead to the weight loss in the Tg mice. Therefore, we assume that the disruption of mitochondorial structure might be caused by cell signaling in which NF90-regulated miRNAs involve. At the moment, we are verifying on this hypothesis.
Dr. Shuji Sakamoto
Education record
Apr, 1997- Mar, 2001: Kochi Medical School, Laboratory of Molecular Biology, Ph.D.’s program
Employment / Research record
Jan, 1999- Mar, 2001: The Japan Society for the promotion of Science, DC
Apr, 2001- Mar, 2002: The Japan Society for the promotion of Science, PD
Apr, 2002- Sep, 2004: Cleveland Clinic Foundation, Lerner Research Institute, Research Fellow
Oct, 2004- Mar, 2007: Kyoto University, Faculty of Medicine, Research Associate
Apr, 2007- present: Kochi University, Kochi Medical School, Science Research Center, Laboratory of Molecular Biology, Assistant Professor
Selected Papers
1. The NF90-NF45 complex functions as a negative regulator in the microRNA processing pathway.
Sakamoto S, Aoki K, Higuchi T, Todaka H, Morisawa K, Tamaki N, Hatano E, Fukushima A, Taniguchi T, Agata Y.
Mol Cell Biol. 2009 Jul;29(13):3754-69.
2. Histone deacetylase activity is required to recruit RNA polymerase II to the promoters of selected interferon-stimulated early response genes.
Sakamoto S, Potla R, Larner AC.
J Biol Chem. 2004 Sep 24;279(39):40362-7.
3. Cells previously desensitized to type 1 interferons display different mechanisms of activation of stat-dependent gene expression from naive cells.
Sakamoto S, Qin J, Navarro A, Gamero A, Potla R, Yi T, Zhu W, Baker DP, Feldman G, Larner AC.
J Biol Chem. 2004 Jan 30;279(5):3245-53.
|
|
|
Signaling mechanism regulating neural tube morphogenesis in ascidians.
Shigeki Fujiwara
Professor, Department of Applied Science, Faculty of Science
Outline of Research
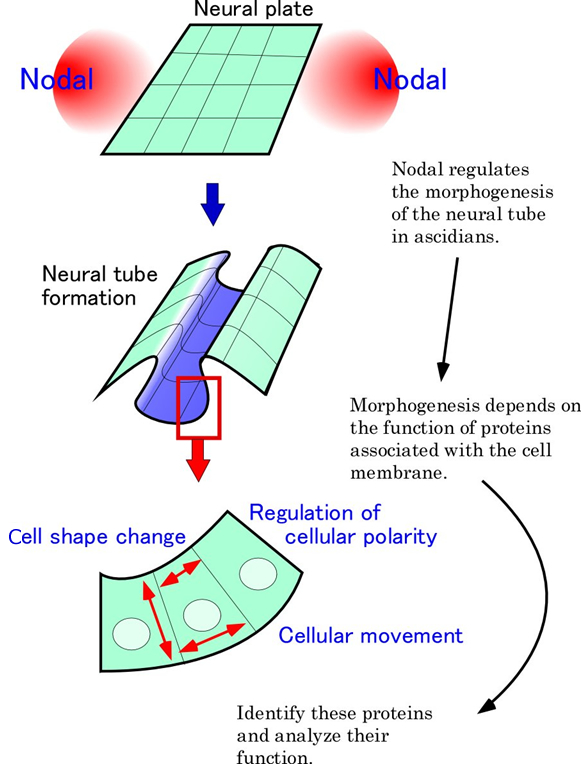
Larvae of ascidians, consisting of only 2500 cells, represent a primitive chordate body plan. Adcidian embryogenesis is an important model system to understand developmental mechanisms and evolution of chordates. I am interested in the morphogenesis of the dorsal neural tube, which is a characteristic feature of chordates. I would like to understand the interaction between extracellular factors (growth factors and the extracellular matrix) and intracellular factors (e.g. transcription factors) during morphogenesis of the neural tube.
Our previous study revealed that the growth factor, Nodal, was involved in the morphogenesis of the neural tube. We also demonstrated that the expression of the transcription factor, Cdx, was activated by Nodal. A dominant-negative form of Cdx disrupted the formation of the neural tube, suggesting that Cdx mediates the Nodal signal during neural tube formation. The target genes of Nodal, identified by our microarray analysis, included many genes encoding extracellular matrix components, cell adhesion molecules, and proteins involved in the regulation of cellular polarity and morphogenesis. Independently, we identified many genes involved in the synthesis and modification of the carbohydrate chains of proteoglycans. Some of these genes are specifically expressed in the neural tube and/or its adjacent organ, notochord.
In this project, functional analysis of these genes will be carried out to understand molecular mechanisms of the morphogenesis of the neural tube. Particularly, I would like to reveal how the receptors and cell adhesion molecules on the cell membrane regulate the function of intracellular factors.
Prof. Shigeki Fujiwara
1983-1987 Kyoto University Bachelor of Science
1987-1990 Kyoto University Graduate School of Science Ph. D. (1993)
1990-1999 Assistant Professor (Department of Biology, Kochi University)
1997-1998 Research Fellow (University of California at Berkeley, Mike Levine laboratory)
1999-2006 Associate Professor (Department of Materials Science, Kochi University)
2006-2008 Professor (Department of Materials Science, Kochi University)
2008-present Professor (Department of Applied Science)
2010-present Adjunct Professor (Department of Marine Biology)
Selected Papers
(1) Epidermal expression of Hox1 is directly activated by retinoic acid in the Ciona intestinalis embryo.
Kanda M, Wada H, Fujiwara S.
Dev Biol. 2009 Nov 15; 335(2): 454-63. Epub 2009 Sep 25. PMID: 19782671
(2) Nodal regulates neural tube formation in the Ciona intestinalis embryo.
Mita K, Fujiwara S.
Dev Genes Evol. 2007 Aug; 217(8): 593-601. Epub 2007 Jul 12. PMID: 17624550
(3) Retinoids and nonvertebrate chordate development.
Fujiwara S.
J Neurobiol. 2006 Jun; 66(7): 645-52. Review. PMID: 16688759
|
|
|
Cell signaling pathways leading to the formation of a resting cyst in protozoa
Tatsuomi Matsuoka
Professor, Laboratory of Animal Physiology, Faculty of Science
Outline of Research
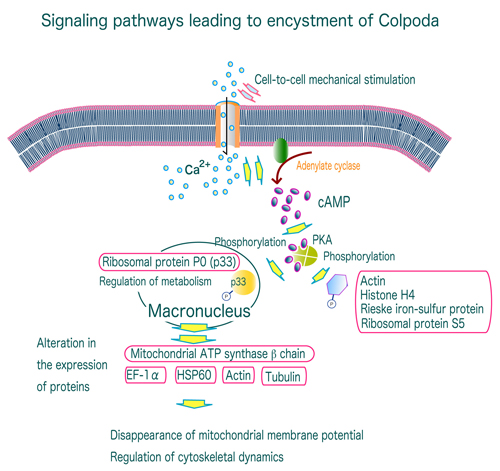
Studies of the molecular mechanisms underlying the formation of resting cysts (including the cysts resistance to desiccation, high temperature, acid, and more) and excystment are important not only from a cell biology viewpoint toward understanding intracellular molecular morphogenetic events, but as fundamental research into the infectious diseases caused by cyst-forming pathogenic protozoa. We have been working on the intracellular signal transduction leading to resting cyst formation and excystment in the soil protozoan Colpoda cucullus. We found that the cyst formation of C. cucullus is mediated by intracellular Ca2+-activated signaling pathways that involve the cAMP/PKA-dependent phosphorylation of several proteins such as actin and ribosomal S5 protein, followed by an alteration of the expression of several proteins such as mitochondrial ATP synthase βchain and heat shock proteins. These results imply that a common molecular mechanism of cyst formation is used by both pathogenic and non-pathogenic protozoa. The our present research focuses on elucidating the function of the proteins involved in the cyst formation by means of feeding RNAi.
Prof. Tatsuomi Matsuoka
Education
1979 Bachelor of Science, Kochi University
1985 Graduate School of Science, Hiroshima University
1986 phD in University of Tsukuba
Career
1986 Postdoctoral fellow, Albert Einstein College of Medicine, USA
1988 Assistant professor, Faculty of Science, Kochi University
1992 Associate professor, Faculty of Science, Kochi University
1999 Professor, Faculty of Science, Kochi University
Award
2000 The Award of the Japan Society of Protozoology
Selected Papers
1) Blepharismin 1-5, novel photoreceptor from the unicellular organism Blepharisma japonicum.
Maeda M, Naoki H, Matsuoka T, Kato Y, Kotsuki H, Utsumi K, Tanaka T.
Tetrahedron Lett. 1997, 38: 7411-7414
2) Protein phosphorylation in encystment-induced Colpoda cucullus: Localization and identification of phosphoproteins.
Sogame Y, Kojima K, Takeshita T, Fujiwara S, Miyata S, Kinoshita E, Matsuoka T.
FEMS Microbiol. Lett. 2012, 331: 128-135.
3) EF-1a and mitochondrial ATP synthase b chain: alteration of their expression in encystment-induced Colpoda cucullus.
Sogame Y., Kojima K., Takeshita T., Kinoshita E., Matsuoka T.
J. Euk. Microbiol. 2012, 59: 401-406.
|
|
|
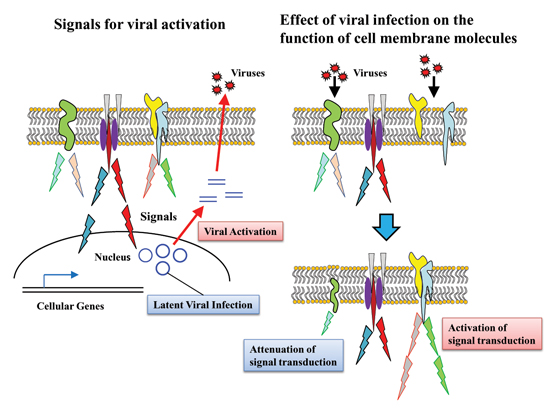
Cell membrane molecules regulating viral gene expression and analysis of their signal transduction
Masanori Daibata
Professor, Department of Microbiology and Infection, Medical School
Outline of Research
Some viruses, such as herpesviruses, establish latent or persistent infection in the host cells, and are reactivated upon a variety of stimuli. Elucidation of mechanism of the viral “latency” and “reactivation” is a very important issue in terms of control of the infectious diseases. Tumor viruses, such as EB virus (EBV), express their own proteins on the membrane of the host cells, thereby generating various signal transductions that lead to cell growth and protection from apoptosis.
In this project, we explore signaling membrane molecules that contribute to the viral reactivation upon
stimuli, focusing on EBV. We also aim at study of the signal transduction leading to expression of the cellular and viral genes. Furthermore, we plan to clarify what membrane molecules are regulated by the microorganism infection.
Prof. Masanori Daibata
Career
1985 M,D., Kochi Medical School, Kochi, Japan
1989 Ph. D. (Medical Science), Graduate School of Medicine,Kochi Medical School, Kochi, Japan
1989-1994 Instructor, Massachusetts Medical School, Worcester, MA, USA
1994-2000 Instructor, Department of Medicine, Kochi Medical School, Kochi, Japan
2000-2003 Assistant Professor, Department of Hematology and Respiratory Medicine,
Kochi Medical School, Kochi, Japan
2003-2007 Assistant Professor, Department of Hematology and Respiratory Medicine,
Medical School, Kochi University, Kochi, Japan
2007-2009 Associate Professor, Department of Hematology and Respiratory Medicine,
Medical School, Kochi University, Kochi, Japan
2009- Professor, Department of Microbiology and Infection, Medical School, Kochi University,
Kochi, Japan
Professional Activities
Fellow of the Japanese Society of Internal Medicine
Board Certified Member of the Japanese Association for Infectious Diseases
Board Certified Member of the Japanese Society of Hematology
Senior Fellow of the Japanese Society of Hematology
Board Certified Member of the Japanese Respiratory Society
Infection Control Doctor (ICD), Japanese College of Infection Control Doctors
General Clinical Oncologist, Japanese Board of Cancer Therapy
Award
2000 Honorable Award (Japanese Cancer Association)
Selected Papers
1. Hashida Y, Imajoh M, Nemoto Y, Kamioka M, Taniguchi A, Taguchi T, Kume M, Orihashi K, Daibata M. Detection of Merkel cell polyomavirus with a tumour-specific signature in non-small-cell lung cancer. Br J Cancer. P108: 629-637, 2013.
2. Daibata M, Nemoto Y, Bandobashi K, Kotani N, Kuroda M, Tsuchiya M, Okuda H, Takakuwa T, Imai S, Shuin T, Taguchi H. Promoter hypermethylation of the bone morphogenetic protein-6 gene in malignant lymphoma. Clin Cancer Res. 13: 3528-3535, 2007.
3. Daibata M, Bandobashi K, Kuroda M, Imai S, Miyoshi I, Taguchi H. Induction of lytic Epstein-Barr virus (EBV) infection by synergistic action of rituximab and dexamethasone renders EBV-positive lymphoma cells more susceptible to ganciclovir cytotoxicity in vitro and in vivo. J Virol. 79: 5875-5879, 2005.
4. Daibata M, Taguchi T, Nemoto Y, Taguchi H, Miyoshi I. Inheritance of chromosomally integrated human herpesvirus 6 DNA. Blood. 94: 1545-1549, 1999.
5. Daibata M, Taguchi T, Sawada T, Taguchi T, Miyoshi I. Chromosomal transmission of human herpesvirus 6 DNA in acute lymphoblastic leukaemia. Lancet. 352: 543-544, 1998.
|
|
|
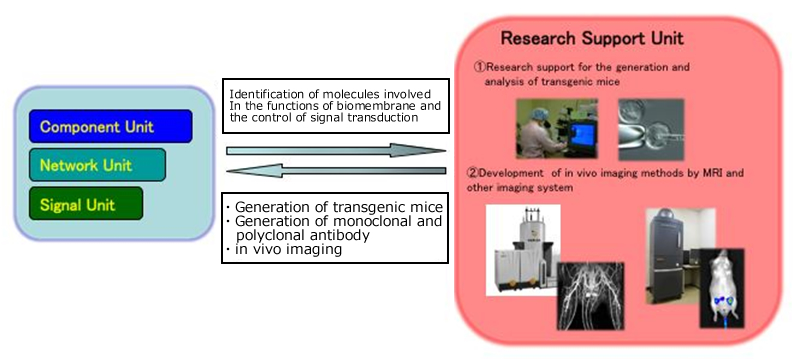
(1) Research support for the generation and analysis of transgenic mice
(2) Development of in vivo imaging methods by MRI and other imaging technologies
Masayuki Tsuda,
Associate Professor, Division of Laboratory Animal Science, Science Research Center
(1) The use of genetically modified mice has played an important role in the life science research. The function of the research support unit is to support investigators through generation of transgenic mice, and monoclonal and polyclonal antibody. In addition, we attempt to develop transgenic mice used as new tools useful to the study of biomembrane and signal transduction.
(2) In our animal facility, in vivo imaging system such as magnetic resonance imaging (MRI) and optical imaging system, was introduced to observe cells, tissues and organs in living animals noninvasively. We are investigating physiological or biochemical roles of biomembrane using these systems, and working to develop new imaging methods.
|
 |
| |
Dr. Masayuki Tsuda
2000.5 Ph.D., Osaka University
2000.6 Post-doctoral fellow, Division of Toxicology, National Institute of Health Sciences
2001.4 Post-doctoral fellow, Department of Mammalian Development, National Institute of Genetics
2005.4 Assistant Professor, Department of Physiology and Biological Information, Dokkyo Medical University
2008.6 Associate Professor, Division of Laboratory Animal Science, Kochi University
Selected Papers
- Tsuda M, Sasaoka Y, Kiso M, Abe K, Haraguchi S, Kobayashi S, Saga Y: Conserved role of nanos proteins in germ cell development. Science. 2003 Aug 29;301(5637):1239-41
- Tsuda M, Kiso M, Saga Y: Implication of nanos2-3’UTR in the expression and function of nanos2. Mech Dev. 2006 Jun;123(6):440-9
- Suzuki A*, Tsuda M*, Saga Y: Functional redundancy among Nanos proteins and a distinct role of Nanos2 during male germ cell development. Development. 2007 Jan;134(1):77-83 (* equal contribution)
|
|





























